When considering the composition of all of the above materials, presence of carbon
as a component element / seems to be a common feature. Carbon is also abundant
in the plants and animals that we find in our environment and all the materials
obtained from those sources.
Elements combine with one another in different ways to create a very large number
of compounds. Quite a majority of them are compounds formed by the combination
of carbon with other elements.
Because of the abundance of carbon containing compounds and the special chemical
characteristics shown by those compounds, carbon chemistry (organic chemistry)
is studied as a separate section under chemistry.
The compounds contaning carbon are commonly referred to as organic compounds
[But, the oxides of carbon, namely carbon dioxide (CO2
) and carbon monoxide (CO) and carbonates and bicarbonates such as sodium carbonate (NaCO3
) and sodium
bicarbonate (Na2
CO3
) are not considered organic]. Organic compounds necessarily
contain carbon and in addition, they may contain elements like hydrogen, oxygen,
nitrogen, halogen, phosphorus and sulphur.
For the convenience of study, organic compounds are classified in various ways.
One method is classifying on the basis of the component elements in the organic
compound. On this basis, the simplest group of organic compounds are hydrocarbons
which contain carbon and hydrogen only.
Compare the list you prepared with the following table.
It is seen that every fuel in the above table contain carbon and hydrogen.
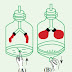
Let us do the following activity to examine whether wax contains carbon and
hydrogen.
This confirms the presence of carbon (C) and hydrogen (H) in candle wax.
All the countries in the world meets their energy requirements by using petroleum
fuels produced by the distillation of crude oil. All compounds in those fuels are
hydrocarbons. Based on the structure, hydrocarbons are classified as alkanes,
alkenes and alkynes.











No comments:
Post a Comment