Heat Transfer
If you touch the far end of a metal spoon inserted in a hot cup of tea you would feel
that it gets warmer gradually. Similarly if you hold your hand above a burning fire,
you would feel that the hand gets warmer. What has happened in these instances is
that heat has transferred along the spoon in the first case and upwards away from
the flame in the second. Heat passing from one place to another in this manner is
known as heat transfer.
Heat transfer occurs from the place with the higher temperature to the place with
the lower temperature. The energy known as thermal energy (heat) of an object
is actually present as the kinetic energy resulting from the random motion of the
particles that form the object. This energy can be the translational, rotational or
vibrational kinetic energy of the particles. Heat transfer is the spreading of kinetic
energy from a region with atoms having a high degree of random motion (with a
high temperature) to a region of atoms having a low degree of random motion (with
a low temperature).
There are three methods of transferring heat.
- Conduction
- Convection
- Radiation
Let us investigate these methods in a simple way.
Conduction
The handle of a metal spoon held in a hot water soon gets warm. Heat passes along
the spoon by conduction.
Some examples where heat transfers by condition are given below.
- Heat flow along a metallic rod in contact with a flame.
- Heat flow from the bottom to the interior of a vessel placed on a cooker.
The main method of heat transfer through solids is conduction.
Since the atoms of a solid are tightly bound to one another, they cannot freely move
throughout the volume of the solid. In such substances, heat exists as the vibrational
kinetic energy of atoms. In metallic substances, part of the thermal energy exists
as kinetic energy of freely moving electrons (free electrons) in addition to this.
Conduction is the spreading of the kinetic energy of atoms and electrons throughout
the substance due to collisions among these particles.
Substances that conduct heat efficiently are known as good conductors and
substances that do not conduct heat efficiently are known as insulators.
Examples: good conductors – silver, copper, iron, mercury, aluminium
Insulators – wood, plastic, asbestos, clay, wool
Existence of free electrons in metals make metals good conductors.
In liquids, molecules are not very tightly bound.
Therefore, conduction of current through liquids
is very weak. Water is a very poor thermal
conductor.This robin has fluffed out its feathers to trap a
layer of air. Air is a poor conductor of heat and
so the bird manages to keep warm even in cold
weather.
Seals, spend all of their lives in cold water. They are protected from losing heat by
conduction by a very thick layer of fat (blubber) which surrounds their body.
Conduction through a metal rod
The Figure illustrates how heat is conducted through a metal rod that is heated
from one end.
Suppose that the metallic rod shown in figure is heated by a flame at the end A.
Then the atoms at that end begin to vibrate with a large amplitude by receiving
thermal energy (heat) from the flame. In addition to this, the free electrons in
random motion at that end gain kinetic energy from the flame. As a result of the
increased kinetic energy, these atoms collide with adjacent atoms. Due to the
collisions, energy transfers to one atom from another increasing the amplitude
of vibrations. This process continues through the atoms in the rod from A to B
in succession, transferring thermal energy along the rod. Thermal energy is also
transferred by the free electrons in random motion in the rod by receiving thermal
energy from the flame.
Convection
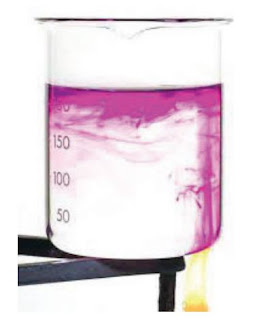
The water is heated just under the purple crystal - the crystal colours
the water as it dissolves. The heated water expands and becomes
less dense than the colder surrounding water, so it floats up to the
top of the beaker. Colder water sinks to take its place, and is then
heated too.
When heat is supplied to liquids or gases they expand and decrease
in density and move upwards. In order to fill these gaps, liquids or
gases with lower temperatures move downwards. Due to this process, heat flows
upwards from the region where heat is supplied. This is known as convection.
When a fire is lighted underneath a tree, branches and leaves above
the fire tend to swing about and burn as a result of the upward
motion of heated up air particles.
Upward motion of heated up particle streams is known as
convection currents.
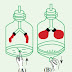
Consider the figures below showing an immersion heater used to
heat up water.
Figure shows a heater partially immersed in water. Here the water near the
bottom of the jug warms up slowly but the water near the top warms up fast. This
happens since convection currents do not flow downwards.
The immersion heater in Figure is fully immersed inside the vessel. Then
water warms up from bottom to top. Heated up water particles become lighter and
move upwards and the water particles that are not heated up move down as their
density is higher. When heated up they too move upwards. This process takes place
continually heating up the whole jug of water.
Formation of Sea breeze and Land breeze
Specific heat capacity of
the earth’s surface in the
land side is smaller than
that of the sea water. Therefore
during day time the
land surface heats up faster
from the sun’s heat than the
sea water. Then the air near
the land surface warms up
which decreases the density
and the air moves upwards.
This reduces the pressure near the ground. Then an air mass flows from the sea to
the land side. This is known as sea breeze.
During night time both the
land and the sea cool down.
The sea cools down slowly
while the land cools down
fast. Air near the sea water
surface is warm while that
above is cold. Therefore
the air near the sea water
moves upwards giving rise
to a low pressure region
just above the sea. Then wind blows from the land side towards the sea in order to
equalize the pressure difference. This is known as land breeze.
Thermal Radiation
You will be able understand that it is not
due to either conduction or convection
that you feel the warmth near a burning
fire. Then it must be through another
means that heat has transferred. We feel
the warmth when thermal rays travel
through space in the form of rays (waves)
from the flame and reach our bodies.
The propagation of heat in the form of electromagnetic radiation from a warm
body without the aid of matter is known as thermal radiation. For heat transfer
by radiation, a material medium is not required. However, for heat transfer by
conduction or convection, particles of a medium are essential.
Heat from the sun reach the earth through a vacuum of about 150 million kilometres
as thermal radiation. Any heated body emits heat as radiation.
Absorption and Reflection of Thermal Radiation
When thermal radiation is incident on an object, part of the radiation is absorbed by
the object while another part is reflected. The surface roughness or the smoothness
and the colour of the surface of the object are the factors affecting the amount of
thermal radiation absorbed or reflected.
Absorption of thermal radiation is high from darker surfaces and rough surfaces.
Reflection of thermal radiation is high from shining surfaces.
Reflection of heat radiation by polished surfaces and by white surfaces are very
high.
Black surfaces absorb a high amount of heat while they reflect a very low amount
of heat.
Situations where thermal radiation is important
When cricketers dress in white during the day time in the presence of sunlight,
the absorption of thermal radiation is very low. Then warming up of the body is
controlled.
Wearing dark colors inside homes by people in cold climates increase the absorption
of thermal radiation. This helps to maintain the body temperature.
If the cooking utensils placed on cookers are black in color, they can absorb thermal
radiation efficiently and transfer heat to the vessels fast.
The inner surfaces of thermos flasks are silvered to make them highly reflective.
These surfaces reflect heat radiated by the contents inside the bottle or heat radiation
coming from outside.















No comments:
Post a Comment