Electronics
Introduction
Electronics has made a huge impact on our daily lives. We use many electronic devices in our day to day activities. Mobile phone, computers, televisions and radios are some examples for such electronic deviceMaterials that conduct electricity are known as electrical conductors. Conductors (copper, aluminium, iron, lead etc.) and mixed conductors (brass, nychrome, manganin) are examples of these. Materials that do not conduct electricity (ebonite, polythene, plastic, dry wood, asbestos, glass etc) are known as electrical insulators.
The reason behind the ability to conduct electricity is the ability of some of the electrons in the atoms of such materials to move freely within the conductor. Electrons in the outer shells of conductors act in this manner since they are not tightly bound to the nucleus. Since inter-atomic bonds (covalent bonds) between the atoms of insulators are strong, there are very few electrons that are free to move.
Meanwhile, some materials conduct a small amount of electricity. Such materials are known as semiconductors. Materials such as silicon (Si) and germanium (Ge) in their crystalline form show such properties. These elements belong to the fourth group in the periodic table and have four electrons in their outermost shell. Such elements form crystal lattice structures by sharing the four electrons in their outermost shell to make covalent bonds with four nearby atoms and thereby acquiring a stable electronic configuration having eight electrons in the outermost shell.
However, these bonds are rather weak and can be broken from the thermal energy available even at room temperature, releasing electrons.
Figure A shows the covalent bonds of the silicon lattice at 0 K. All the bonds are complete at this temperature. Figure B shows that some bonds have been broken releasing some free electrons at a temperature higher than 0 K. An electron deficiency can be observed at the positions that the free electrons occupied previously. Such positions with an electron deficiency are known as holes. Due to the positively charged protons in the nucleus, a hole gives rise to a positive charge that has not been neutralized (In a neutral atom, the number of protons in the nucleus is equal to the number of and electrons). Therefore a hole is equivalent to a positive charge.
Figure A
A silicon lattice at 0 K
Figure B
A silicon lattice at temperature above 0 K
while holes act as positive charge carriers.
Therefore, when an electric potential difference is applied across a semiconductor, holes move from the positive to the negative potential while electrons move from the negative to the positive potential and the (conventional) current flows from the positive to the negative potential.
- In metallic conductors, the charge carriers that conduct electricity are the negatively charged electrons.
- In semiconductors, the negatively charged electrons as well as the positively charged holes act as the charge carriers that contribute in the conduction of electricity.
- Since a hole is generated in the breaking of a bond to release an electron, the number of carrier electrons present in a semiconductor is equal to the number of holes.
- Therefore the semiconductor lattice is electrically neutral.
Intrinsic Semiconductors
Pure semiconductor materials such as silicon (Si) and germanium (Ge) that exist in crystaline form as mentioned above are known as intrinsic semiconductors.
Effect of Temperature on the Conduction of Electricity
Since the random motion of free electrons increases as the temperature is increased, a rise in the temperature inhibits the current flow. Therefore, a temperature rise in conductors causes a decrease in the conductivity (increase in the resistivity). However in semiconductors, a rise in temperature breaks bonds generating more holes and free electrons causing an increase in the conductivity (decrease in the resistivity).
Extrinsic Semiconductors
Let us consider what happens when a minute amount of the element phosphorous (P) is mixed (doped) to an intrinsic semiconductor such as Si. Phosphorous is an element in group V of the periodic table and has five electrons in the outermost shell. A phosphorous atom makes the number of electrons in its outermost shell eight by acquiring four electrons from four nearby silicon atoms around it. In the process, one of the five electrons is left behind without taking part in forming a
bond. This electron has the opportunity to move about freely in the lattice.
Figure C
A Si lattice doped by phosphorous
Figure C shows how a phosphorous atom forms bonds with silicon atoms. The
electron left behind increases the conductivity of the lattice. Since negatively
charged electrons are introduced to the lattice as charge carriers, the semiconductor
is known as a negative type or n-type semiconductor. Semiconductors whose
carriers have been increased by doping it with another element are known as
extrinsic semiconductors. By doping an intrinsic semiconductor with other elements in group V such as arsenic (As) and antimony (Sb) also, n-type extrinsic semiconductors can be formed. Since electrons are donated to the lattice by group V elements, they are known as donor atoms.
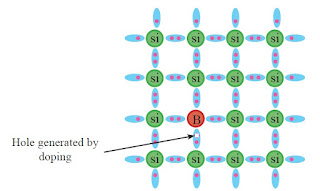
If Si which is an intrinsic semiconductor is doped with an element in group III such as boron (B), the boron atom forms bonds with nearby silicon atoms. However, since there are only three electrons in the outermost shell of the boron atom, there is a deficiency of one electron in order to form four bonds. Figure D shows how the atoms and bonds are configured in this case.
Figure D
A Si lattice doped by boron
A hole exists at the point where the electron is deficient in the boron atom to form a bond. Since holes can conduct electricity as positive charges, the conductivity of silicon increases. As a hole is equivalent to a positive charge, such extrinsic semiconductors are known as positive or p-type semiconductors. By doping an intrinsic semiconductor with other elements in group III such as aluminium (Al), gallium (Ga) and indium (In) instead of B also p-type extrinsic semiconductors
can be formed. Since holes that can receive electrons are produced by group III elements, they are known as accepter atoms.
source by internet and books











No comments:
Post a Comment