Heat
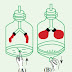
Let us put equal volumes of water into two identical vessels at room temperature. Next let us insert two thermometers and arrange the set up above two candles as shown below Figure Now let us light up the candle in Figure (b) while leaving that in Figure (a) as it is.The temperature of the water in Figure (a) remains unchanged. The temperature of the water in Figure 9.8(b) can be seen to increase gradually.
Only the candle in Figure (b) has been lighted. Therefore the temperature of water in that vessel has increased. From this it is clear that something has transferred from the candle flame to the water and that the temperature of the water has risen as a result of it. Here, heat has transferred to the water.
Therefore, the energy transfers from one object to another as a result of the temperature difference existing between the two objects is known as the heat.
Heat Transfer
Let us investigate what happens when we put a heated piece of iron into a cold water vessel.
Activity 01
Apparatus required:
A heated block of iron, A thermometer, A stirrer, A vessel with water at room temperature.
Put a heated piece of iron into a cold water vessel.
Observe the temperature of the water.
You will observe that the temperature of the water rises.
What happens here is the flow of heat from the iron which is at a higher temperature into the water which is at a lower temperature.
As the temperature of the water increases, the vessel also heats up as a result of absorbing heat. As heat flows out from the iron block, its temperature gradually decreases. After a while, the temperatures of the water and the iron block become equal. After reaching this common temperature, heat does not flow to the water from the iron block or to the iron block from the water. This state is known as thermal equilibrium. Just as water flows from a higher level to a lower level, heat also flows from a body at a higher temperature to a body at a lower temperature.
Therefore,
- Heat transfers from a body at a higher temperature to a body at a lower temperature.
- Then the temperature of the body at the lower temperature increases.
- At the same time, the temperature of the body at the higher temperature decreases.
Since heat is a form of energy, heat can be measured in Joules (J). The international unit for measuring heat is the Joule. In addition to this, the unit known as the Calorie is also frequently used to measure heat (thermal energy).
Heat Capacity of an Object
Activity 02
Apparatus required : Three identical beakers, Water, Coconut oil, Threethermometers, Three identical candles
- Obtain three identical beakers and pour a measured volume of water into one of them.
- Pour an equal volume of coconut oil into another beaker.
- Pour water with a volume equal to twice the initial volume into the third beaker.
- Measure the temperatures of the liquids in all three beakers.
- Now place all three beakers on three identical stands and heat them up for an equal time interval (about 5 minutes) using three identical candles.
- At the end of the time interval measure the temperatures of the liquids.
Even though there could be minor differences in the candles, we could assume that approximately the same amount of heat was supplied to each of the three beakers. However you will observe that the temperature rise in the three beakers are different.
You will understand from this activity that when the same amount of heat is supplied to different quantities of the same substance or the same quantities of different substances, their temperatures rise in different amounts.
Since the temperature rise in the three beakers of the above activity were not equal although the same amount of heat was supplied to all three beakers, we can conclude that the heat capacities of the substances in the three beakers are different.
The amount of heat required to increase the temperature of an object by one unit is known as the heat capacity of the object.
- The international unit for measuring heat capacity is Joules per Kelvin (J K-1).
- Heat capacity can also be expressed in Joules per degree Celsius (J oC-1).
The heat capacity of an object depends on the substance that the object is made of and it’s mass. Two objects made out of the same substance but with different masses have different heat capacities. Even though the masses are the same, two objects made out of different substances can have different heat capacities. The heat capacity of a substance is indicated by the symbol C.
Specific Heat Capacity
It can be experimentally shown that the heat capacity of different masses of the same substance is proportional to the mass. This means that the heat capacity doubles when the mass is doubled. However the heat capacity of a unit mass of a given substance or the amount of heat required to increase the temperature of a unit mass of the substance by one degree is a property that depends on the substance.
The amount of heat required to increase the temperature of a unit mass of a given substance by one degree is known as the specific heat capacity of the substance.
Since the specific heat capacity is the amount of heat that should be supplied to increase the temperature of a unit mass of a given substance by one degree, it can also be described as the heat capacity of a unit mass. Therefore, the heat capacity of an object can be obtained by multiplying the specific heat capacity of an object by its mass.
Units of specific heat capacity is J kg-1 K-1 (Joules per kilogramme per Kelvin) or J kg-1 oC-1 (Joules per kilogramme per degree Celsius).
The specific heat capacity of a substance is indicated by the symbol c.
source by internet and books













No comments:
Post a Comment