Types of mixtures
Let us pay our attention to the composition of the air around us. Air is composed of gases like nitrogen, oxygen, argon and carbon dioxide, water vapour, and very small particles such as dust. So, you may understand that air is a mixture of several substances.Therefore, if some matter contains two or more substances, such matter is referred to as mixtures. You have already learnt that elements and compounds are pure substances. But, mixtures are not pure substances. Natural environment mostly contains mixtures, not pure substances. Air, soil, sea water, river water and rocks around us are examples. The things that we drink such as cool drinks, fruit drinks, tea, coffee and food items such as ice cream, yoghurt and fruit salad are also mixtures. Let us do the following activity to study more about the components of a mixture.
Activity 01
Materials required; - Hydrated copper sulphate, naphthalene, (moth balls), mortar and pestleMethod; - Take some copper sulphate and naphthalene (moth balls) into a mortar, grind them together with the pestle into a powder and mix well. Transfer the powder onto a piece of paper and observe.
At a glance, you may not be able to see two substances, copper sulphate and naphthalene but, you have a mixture of the two. A blend of two or more pure substances is called a mixture and individual substances that form the mixture are known as components.
Activity 02
Materials required ;- Two beakers, a glass rod, a funnel, a filter paper, hand lens.Method ;- Transfer the mixture made in activity above in to a small 100 ml beaker, add about
50 ml of water to it and stir well. Then, place a filter paper in a funnel, glass and filter this solution into another beaker. Allow the residues on the filter paper to dry and observe with a hand lens. Observe the filtrate as well.
From this activity, you would have understood that the remains on the filter paper is naphthalene powder. Since the solution is blue in colour, the substance that dissolved in water is copper sulphate.
The above activity clarifies another feature of a mixture. That is, even when the components are in a state of being mixed, their chemical nature remains unchanged. In other words, the identity of the components constituting a mixture does not change even in the mixture. Moreover, the above activity shows that the components in a mixture can be separated by physical methods.
Thus mixtures can be defined as follows: Mixtures are matter consisting of two or more components which are not chemically combined, and can be separated by physical methods.
Mixture Components
Cement mortar Sand, cement, water
Cake Sugar, flour, water, colouring, butter
Well water Water, dissolved oxygen, dissolved carbondioxide
and various salts.
Sea water Water dissolved oxygen and salts such as sodium
chloride, magnesium chloride, magnesium sulphate,calcium sulphate
When considering mixtures, it is very important that how well the components are mixed. Understand this thoroughly with the help of the following examples.
Ex:-
- When making colours by mixing paints, application of the paint will not give a uniform colour unless they are mixed well.
- If the components used to make cake are not mixed well, different parts of the cake taste differently. Also, the rising will be different in different parts.
- The medicinal property of tablets, capsules or liquid mixtures is not even in all the parts if the components are not mixed well when producing medicines.
Investigate into more instances like the above examples.
Activity 03
Materials required ;- A beaker, clay, water, a piece of clothMethod ;-
(i) Take about 500 ml of water into a beaker. Add about 10g of clayey soil to it, stir well and allow to stand still for about one minute. Then filter the muddy coloured water into another beaker using a piece of cloth. Allow to stand still for about an hour and see whether the muddy colour is uniformly distributed throughout the solution. See if the clearness of the solution is similar from top to bottom.
(ii) Take a piece of a metal sheet with a lustrous surface. As shown below figure, take two identical drops of the solution from two places A and B with a pipette or glass rod, place them on the spots marked as A and B respectively, on the piece of metal and let them vaporize. Check to see which water sample contains more residual matter, See, water obtained from which place contains more residual matter.
The above activity leads to the following conclusions. In the mixture formed by dissolving clay in water,
- The colour/transparency is different from place to place.
- The amount of clay particles in a unit volume is different from place to place.
Activity 04
Materials required ;- A beaker, water, common salt, a piece of cloth
Method ;- Take about 250 ml of water into a beaker add about 10 g of pure salt into it, stir till the salt dissolves and filter the solution with a piece of cloth. Allow to stand still for about one hour and see whether the clearness of the solution is equal from top to bottom. Repeat what you did in activity 02 for this solution as well.
Following conclusions can be drawn from the above activity. In the mixture formed by dissolving salt in water,
- The transparency is equal throughout the solution.
- The amount of salt particles in a unit volume of the solution is equal throughout the solution.
Pay your attention again to the mixtures you studied in activities 03 and 04. According to the nature of the distribution of components, mixtures can be divided in to two categories.
- Mixtures in which the composition of the components is uniform throughout the mixture. Ex: mixture prepared by dissolving common salt in water.
- Mixtures in which the composition of the components is not uniform throughout the mixture. Ex: mixture prepared by dissolving clay in water
The mixtures in which the components are separated from one another are called heterogeneous mixtures. The mixtures whose composition of the components are uniformly distributed throughout are known as homogeneous mixtures.
Homogeneous mixtures
The mixtures in which the components cannot be observed separately from one another and the properties and composition are similar throughout are termed homogenous mixtures. In a homogeneous mixture, the physical properties such as colour, transparency and density are identical everywhere. Homogenous mixtures are also known as solutions.
Examples: salt solution, sugar solution
Heterogeneous mixtures
The mixtures in which the components can be distinguished from one another and their are known as a heterogeneous mixtures. The physical properties of the mixture such as colour, transparency and density are different from place to place, in a heterogeneous solution.
Examples: Water in which clay is dissolved, water in which laundry blue (the powder used for whitening of clothes) is dissolved, cement mortar, sherbet drinks, fruit salad
Classify the various mixtures you prepared as homogeneous and heterogeneous
Heterogeneous and homogeneous mixtures can be classified further depending on the physical state of the components. Study and understand the facts given in below table describing mixtures of two components.
Activity 5
Dissolve the following substances in water and record the observations. Common salt, washing powder, laundry blue (added to clothes), copper sulphate, potassium permanganate, wheat flour, ethyl alcoholClassify the various mixtures you prepared as homogeneous and heterogeneous
Heterogeneous and homogeneous mixtures can be classified further depending on the physical state of the components. Study and understand the facts given in below table describing mixtures of two components.
* Brass is an alloy composed of 65% of copper and 35% of zinc. This is a solid-solid homogeneous mixture.
Solute and solvent of a solution
It was mentioned that a homogenous mixture is also called a solution. A solution is composed of a solvent and one or more solutes. Of the components mixed to form the solution, the component present in excess is the solvent. The rest of the components are solutes.
Hence, it can be represented as,
Ex: Salt + Water =Salt solution
Copper sulphate + Water = Copper sulphate solution
Sugar + Water = Sugar solution
Solubility of a solute
What will happen if a small amount of a given solute is added to a solvent? It will dissolve and disappear.
What quantity of a solute can be dissolved like this in a given volume of the solvent?
Do the following activity to find it out.
Method ;- Measure 100 ml of water into a clean beaker. Weigh 100 g of pure salt (NaCl). Add salt, little at a time into the beaker of water and stir with the glass rod to dissolve it. Do not add more salt until the salt added before dissolves completely. When no more salt dissolves, stop adding more salt and weigh the remaining amount of salt. Approximately, What is the maximum mass of salt that
can be dissolved in 100 ml of water?
Is the amount of other components that dissolve in the same volume of water, the same? To investigate this, conduct the following activity.
Method ;- Weigh 10 g of calcium hydroxide at the laboratory. Take 100 ml of water to a beaker and dissolve calcium hydroxide in it, by adding a very small amount at a time while stirring. Stop the addition of solid when no more calcium hydroxide dissolves in solution and weigh the remaining solid. Approximately what is the maximum mass of calcium hydroxide that can be dissolved in
100 ml of water?
Compare the results of the activity 7 with those of 6
The above activities show that the quantity dissolved is more for some substances, while it is less for same other substances in the same volume of water.
Repeat activities 6 and 7 using 100 ml of hot water of about 80 0C instead of water at room temperature. See whether the dissolved mass of the solute changes. It would be observed that a greater amount of the solute dissolves at a higher temperature than it does in an equal volume of water at room temperature.
In order to compare the dissolution of various solutes in a given solvent, the amount of solutes dissolved in the same volume of the same solvent at the same temperature should be compared. Therefore solubility is defined as follows.
The solubility of a solute at a given temperature can be defined as the mass of that solute which dissolves in 100 g of the solvent at a certain temperature.
Ex:- at 25 0C, the solubility of magnesium chloride in water is 53.0 g.
At the same temperature, solubility of potassium sulphate in water is 12.0 g.
Factors affecting solubility
You have already identified temperature as a factor affecting the solubility of a solute in a given solvent. Try the following activities to investigate the other factors affecting solubility.
Method ;- Take 50 ml of water at the same temperature into each of two small beakers. Accurately Weigh 50 g each of salt and sugar. Adding a little at a time, dissolve salt in one beaker and sugar in the other. When it comes to the point beyond which no more solid dissolves, stop adding the substance and weigh the remaining solid. See whether the amounts left are equal.
You will be able to see that at the same temperature, different solutes dissolve in different amounts in equal volumes of the same solvent. Hence, it can be said that the nature of the solute affects the solubility.
Method ;- Take 50 ml each of the solvents water and kerosene at the same temperature into two small beakers. Add 5 g of sugar into each of them and stir. In which solvent does sugar dissolve?
You will be able to see that the sugar completely dissolves in water but sugar added to kerosene remains almost undissolved.
This shows that the solubility of the same solute is different in equal volumes of different solvents at the same temperature.
Therefore, it can be said that the nature of the solvent affects solubility.
According to the observations of the above activities, it is confirmed that the following factors affect solubility.
1. Temperature
2. Nature of the solute
3. Nature of the solvent
Of the above factors, except temperature, the nature of the solute or the solvent are properties of matter. The characteristic properties of matter are imparted by the particles that make the matter. The nature of molecules which constitute the solute and solvent is a factor that determines the solubility. In grade 10 you have learnt about the polarity of a chemical bond. Based on the polarity chemical compounds can be classified into two categories; polar and non polar. At the same time, chemical
compounds can also be classified into two types organic and inorganic - according to the constituent elements of the compound.
Hence, solutes and solvents can be classified under four classes
1. Polar organic solutes/solvents
2. Non - polar organic solutes/ solvents
3. Polar inorganic solutes/solvents
4. Non - polar inorganic solutes/solvents
From the following schematic diagram, you can identify the examples relevant to those four classes.
Based on the above classification, a general concept on solubility such as the one below can be composed.
Polar solutes are soluble in polar solvents
Ex 1 :- Ethanol is a polar compound. Water is a polar compound. Therefore, ethanol is soluble in water.
Ex 2 :- Ammonia is a polar compound. Water is a polar compound. Therefore, ammonia dissolves in water.
Non-Polar solutes are soluble in non-Polar solvents
Ex 1:- Grease is a non-polar solute. Kerosene is a non-polar solvent. Therefore, grease dissolves in kerosene.
Ex 2:- Jak glue (koholle) is a non-polar solute. Kerosene is a non-polar solvent. Therefore, jak glue is soluble in kerosene.
On that account, it can be concluded that solutes and solvents of similar polarity properties dissolve in each other (like dissolves like).
Solubility of a gas
Do gases really dissolve in water? Recall the following experiences to answer this.
Solute and solvent of a solution
It was mentioned that a homogenous mixture is also called a solution. A solution is composed of a solvent and one or more solutes. Of the components mixed to form the solution, the component present in excess is the solvent. The rest of the components are solutes.
Hence, it can be represented as,
Solvent + Solute = Solution
This can be further understood by paying attention to the solutions used in day-to-day life.Ex: Salt + Water =Salt solution
Copper sulphate + Water = Copper sulphate solution
Sugar + Water = Sugar solution
Solubility of a solute
What will happen if a small amount of a given solute is added to a solvent? It will dissolve and disappear.
What quantity of a solute can be dissolved like this in a given volume of the solvent?
Do the following activity to find it out.
Activity 6
Materials required ;- A beaker, salt, a glass rodMethod ;- Measure 100 ml of water into a clean beaker. Weigh 100 g of pure salt (NaCl). Add salt, little at a time into the beaker of water and stir with the glass rod to dissolve it. Do not add more salt until the salt added before dissolves completely. When no more salt dissolves, stop adding more salt and weigh the remaining amount of salt. Approximately, What is the maximum mass of salt that
can be dissolved in 100 ml of water?
Is the amount of other components that dissolve in the same volume of water, the same? To investigate this, conduct the following activity.
Activity 7
Materials required ;- A beaker, calcium hydroxide, a glass rodMethod ;- Weigh 10 g of calcium hydroxide at the laboratory. Take 100 ml of water to a beaker and dissolve calcium hydroxide in it, by adding a very small amount at a time while stirring. Stop the addition of solid when no more calcium hydroxide dissolves in solution and weigh the remaining solid. Approximately what is the maximum mass of calcium hydroxide that can be dissolved in
100 ml of water?
Compare the results of the activity 7 with those of 6
The above activities show that the quantity dissolved is more for some substances, while it is less for same other substances in the same volume of water.
Repeat activities 6 and 7 using 100 ml of hot water of about 80 0C instead of water at room temperature. See whether the dissolved mass of the solute changes. It would be observed that a greater amount of the solute dissolves at a higher temperature than it does in an equal volume of water at room temperature.
In order to compare the dissolution of various solutes in a given solvent, the amount of solutes dissolved in the same volume of the same solvent at the same temperature should be compared. Therefore solubility is defined as follows.
The solubility of a solute at a given temperature can be defined as the mass of that solute which dissolves in 100 g of the solvent at a certain temperature.
Ex:- at 25 0C, the solubility of magnesium chloride in water is 53.0 g.
At the same temperature, solubility of potassium sulphate in water is 12.0 g.
Factors affecting solubility
You have already identified temperature as a factor affecting the solubility of a solute in a given solvent. Try the following activities to investigate the other factors affecting solubility.
Activity 8
Materials required ;- Two small beakers, common salt, sugarMethod ;- Take 50 ml of water at the same temperature into each of two small beakers. Accurately Weigh 50 g each of salt and sugar. Adding a little at a time, dissolve salt in one beaker and sugar in the other. When it comes to the point beyond which no more solid dissolves, stop adding the substance and weigh the remaining solid. See whether the amounts left are equal.
You will be able to see that at the same temperature, different solutes dissolve in different amounts in equal volumes of the same solvent. Hence, it can be said that the nature of the solute affects the solubility.
Activity 9
Materials required ;- Two small beakers, kerosene, sugarMethod ;- Take 50 ml each of the solvents water and kerosene at the same temperature into two small beakers. Add 5 g of sugar into each of them and stir. In which solvent does sugar dissolve?
You will be able to see that the sugar completely dissolves in water but sugar added to kerosene remains almost undissolved.
This shows that the solubility of the same solute is different in equal volumes of different solvents at the same temperature.
Therefore, it can be said that the nature of the solvent affects solubility.
According to the observations of the above activities, it is confirmed that the following factors affect solubility.
1. Temperature
2. Nature of the solute
3. Nature of the solvent
Of the above factors, except temperature, the nature of the solute or the solvent are properties of matter. The characteristic properties of matter are imparted by the particles that make the matter. The nature of molecules which constitute the solute and solvent is a factor that determines the solubility. In grade 10 you have learnt about the polarity of a chemical bond. Based on the polarity chemical compounds can be classified into two categories; polar and non polar. At the same time, chemical
compounds can also be classified into two types organic and inorganic - according to the constituent elements of the compound.
Hence, solutes and solvents can be classified under four classes
1. Polar organic solutes/solvents
2. Non - polar organic solutes/ solvents
3. Polar inorganic solutes/solvents
4. Non - polar inorganic solutes/solvents
From the following schematic diagram, you can identify the examples relevant to those four classes.
Based on the above classification, a general concept on solubility such as the one below can be composed.
Polar solutes are soluble in polar solvents
Ex 1 :- Ethanol is a polar compound. Water is a polar compound. Therefore, ethanol is soluble in water.
Ex 2 :- Ammonia is a polar compound. Water is a polar compound. Therefore, ammonia dissolves in water.
Non-Polar solutes are soluble in non-Polar solvents
Ex 1:- Grease is a non-polar solute. Kerosene is a non-polar solvent. Therefore, grease dissolves in kerosene.
Ex 2:- Jak glue (koholle) is a non-polar solute. Kerosene is a non-polar solvent. Therefore, jak glue is soluble in kerosene.
On that account, it can be concluded that solutes and solvents of similar polarity properties dissolve in each other (like dissolves like).
Solubility of a gas
Do gases really dissolve in water? Recall the following experiences to answer this.
- As soon as a bottle of soda water or a fizzy drink is opened, gas bubbles evolve from the solution.
- When a beaker of water is heated, gas bubbles can be seen on the walls of the beaker.
In both of these instances, it is the gases that were dissolved in water that got liberated. During the production of soda water, carbon dioxide gas is mixed with water under the special conditions of high pressure using machinery. Because of this more gas dissolves in water. However, atmospheric gases are always in contact with natural water. Then gases like carbon dioxide and oxygen dissolve only in
small quantities.
When water is heated, the dissolved gases are evolved. Therefore, the amount of gases remaining dissolved in hot water is very small. Accordingly, temperature can be taken as a factor affecting the solubility of a gas.
Generally, the solubility of a solid substance in a solvent can be increased by increasing the temperature. But the solubility of a gas in a given solvent decreases with the rise in temperature. Are there any more factors affecting the solubility of a gas in water? See what conclusion could be drawn from the observations of the following activity.
Activity
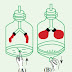
Materials required ;- an unopened bottle of soda water (plastic), an empty bottle of the same type.
Method ;- Take an un opened bottle of soda water available in the market. To an identical empty bottle, add water equal in quantity to that of soda water and close the cap tightly. Now squeeze both bottles with hand and select the harder bottle.
You may feel that the unopened soda bottle is very hard that it cannot be pressed. Think why it is so. In the bottle of soda above the liquid, there is carbon dioxide gas under high pressure. The moment the cap is opened, carbon dioxide gas escapes, and the rigidity of the bottle is lost. When the pressure of a gas in contact with water is increased, the solubility of that gas in water too increases. Thus, the solubility of a gas in water depends on two factors.
1. Temperature
2. Pressure
source by internet and books















No comments:
Post a Comment